Limescale: what is and how is it formed?
Limescale is the number one enemy of household appliances, surfaces, sanitary and, above all, savings.
Read on to find out what it is, how it is formed and how to eradicate it.
And don’t miss the surprise at the end of the article!
WATER PURIFICATION
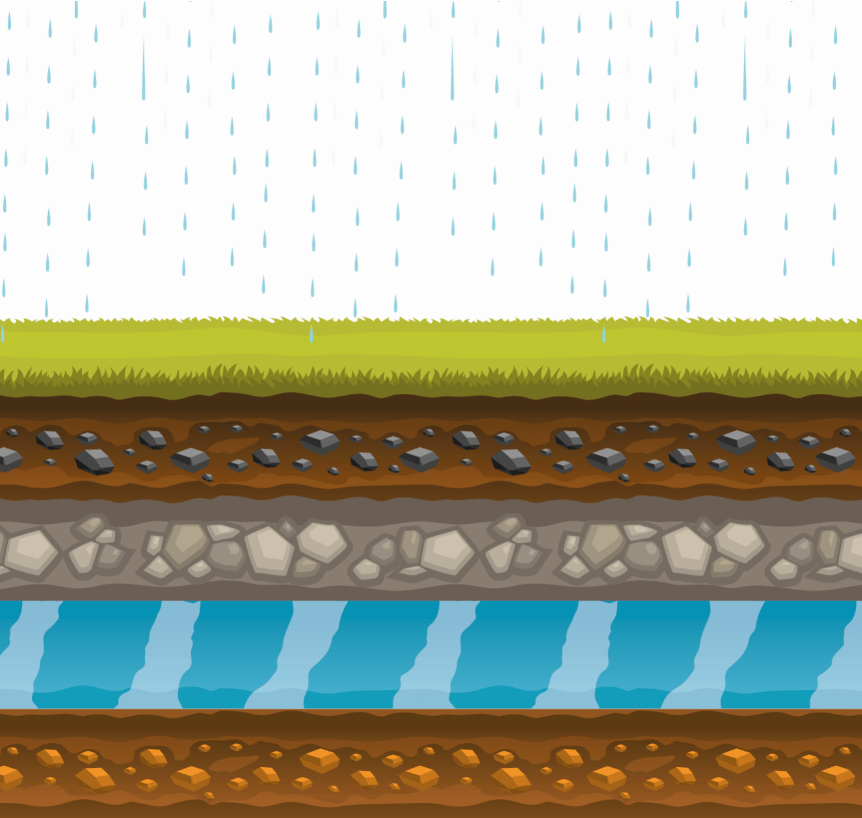
The water supply can be of superficial or underground origin.

Surface waters (basins, reservoirs, rivers, etc.) present serious problems when they must be purified as they incorporate the gases they encounter in the atmosphere when they fall in the form of rain, snow or hail, as well as all the pollutants that man has distributed in the soil.
Groundwater is deep water that has leaked into the ground.
This percolation causes the water to be filtered and, in this way, the soil absorbs all the pollutants that the water has, in turn, absorbed during precipitation and contact with the soil.
This makes water purification easier also due to the fact that these waters are not influenced by atmospheric agents and do not change their chemical and physical characteristics.
WATER HARDNESS
The unit of measurement of hardness is the French degree °f and each French degree corresponds to 10 mg/L of calcium carbonate (scale).
If the water hardness is 30 °f it means that 300 grams of limescale are deposited in the water system every 1000 liters of water consumed.
HOW LIMESCALE IS FORMED

Limescale forms when calcium and magnesium, bound to bicarbonate, precipitate due to changes in temperature.
If there were no bicarbonates, the water would create encrustations only at its boiling temperature.
Given the huge amount of carbon dioxide that every day is emitted into the atmosphere all the calcium and the present magnesium binds with bicarbonate.
Calcium and magnesium bicarbonate are very unstable soluble salts: small changes in temperature and pH are enough to transform the calcium and magnesium bicarbonate into calcium and magnesium carbonates which, being insoluble in water, cause the scale.
HOW ENCRUSTATIONS ARE FORMED
As previoulsy explained, the water, depending on its characteristics, can cause encrustations (calcium and magnesium carbonate deposits) even with small variations in temperature and flows.

When the water passes or stops on metal components, the phenomenon is accentuated, as the crystalline structure of the metal is not as smooth as a plastic material, but much more rough.
This roughness causes the crystalline germ of calcium carbonate to cling to it and, consequently, due to electrochemical forces (limestone attracts limestone) the encrustation originates.
It is, above all, when the water is heated that most of the limestone tends to deposit!

Just think about what happens when you prepare a tea:
You put the water in the pot and light the fire. Even if after a few minutes the water has not yet reached the boiling temperature, you can still notice the formation of small bubbles.
What are these bubbles?
They are nothing more than the carbon dioxide that is released from the decomposition of bicarbonate into carbonate.
After a few minutes you will see a white deposit forming on the edge of the pot in contact with the water.
This, however, is what happens when we take a shower: the boiler heats water, the carbon dioxide that is released comes out of the shower head and it is there, where the heat exchange takes place, that limestone tends to settle more.

Now imagine preparing 200 teas, always with the same pot without ever washing it: 200 teas at 200 cc per tea, make 40 liters of water and that is the same amount that we consume for a shower.
Can you imagine how much the heat exchanger becomes encrusted after 100 showers?
WATER TREATMENT
Considering what was said earlier, it is necessary to treat the water precisely to avoid the inconveniences in terms of energy costs and environmental impact which are no longer justified for a country like Italy.
The Italian Interministerial Decree of 26/06/2015 requires the treatment of water for the production of domestic hot water and heating water, regardless of the hardness and thermal capacity of the system.
There are two types of treatment to solve and prevent the problem:
PHYSICAL TREATMENT
By physical treatment we mean all those devices capable of creating a magnetic field able of breaking the calcium carbonate crystal (which has the shape of a six-pointed star) into many small parallelepipeds, which hardly stick to the walls.
We are talking about permanent magnets, electromagnet or current equipments.
However, there are many contraindications that most of times invalidate the result of these devices to the point that the Interministerial Decree does not even take them into consideration
CHEMICAL TREATMENT
There are two types of Chemical treatments:
– The dosage of chemical products that prevent the deposit of limescale, which consists in adding, in proportion to the flow of water, a substance that is able to surround (chelating action) the calcium and magnesium ion, making it slippery and, consequently, preventing its precipitation. This treatment is mandatory for all types of domestic hot water production systems, even for the simple wall-mounted boiler.
– The almost complete elimination of calcium and magnesium, through resins that have the characteristic of retaining these ions and then discharge them when it is time.
WHAT TO DO IF THE RIGHT PREVENTION HAS NOT BEEN DONE?
If you have hard water and you haven’t done the right prevention with chemical systems, sooner or later you will have a limescale deposit which will prevent the use of encrusted equipment.
The most common case is the flow rate of hot water which is so reduced by encrustations, that it will be impossible to even take a hot shower or, even, the boiler will lock out.

At this point it is necessary to intervene with
DESCALING
to restore the operating conditions of the boiler.
Descaling is an acid wash to be carried out with acid-proof pumps. The type of acid to be used depends on the types of metals with which it comes into contact.
Do you want to discover the procedures for descaling?
Download our
“Technical Book Descaling”